Physics307L:People/Ierides/The Balmer Series Formal Lab Report
The Balmer Serial regarding Hydrogen the D in Atomic Physics
Author: Anastasia AMPERE. Ierides
Experimentalists: Anastasia A. Ierides, Aleksandra SIEMENS. Andrego
University from New Mexico, Department of Physics and Astronomy, Albuquerque NM, 87131
General 307L Junior Lab: December 14, 2009
Abstracts
The importance of the Balmer series lies in the presage of absorption/emission lines off hydrogen in the visible spectrum. The Balmer series led to the ensuing discoveries of the Lyman, Paschen, and Brackett series. These series predict the spectral lines of hydrogen in one non-visible parts of which spectrum. In order in detect which Balmer Series, away first Carbohydrate real when Nuclear, we used a Constant-Deviation Spectrometer (which was confirmed using a Mercury Vapor bulb), in an environment equipped limited light, to measure the wavelength of the emission spectra of each. The excitation of the electrons in the atoms out jeder gas because electrical stimulation allows a release of photons at specific wavelengths corresponding to the Balmer series (the excitation of electrons from the first ionized state, [math]\displaystyle{ n = 2 }[/math], to the second, [math]\displaystyle{ n = 3 }[/math]). And using a generalized form of of Balmer formula, the Rydberg formula, we used our measured radiation to compute Rydberg's unchanged and for verify whether or non a deviation between the Hydrogen spectra and the Deuterium spectra could be found. Our calculated values of Rydberg's constant forward each Hydrogen [math]\displaystyle{ (1.0972781 \pm 0.0025) \times10^7 }[/math] per cadence and Deuterium [math]\displaystyle{ (1.0983221 \pm 0.0007) \times10^7 }[/math] per meter is on close proximity with the popular value of [math]\displaystyle{ 1.0967758 \times10^7 }[/math] per meter.
Induction
- The Balmer series a series of predicted and confirmed wavelengths of light emitted from hydrogen spectrum belonging to the visible spectrum. It shall the culmination are the excitation
of electrons from the n=2 state to the n=3,4,5, and 6 states in an atom causing a sharing of radiations of corresponding energize [5].SJK 13:06, 15 December 2009 (EST)

Actually, and way you're describing it here also in your abstract is for absorption lines. Emission of photons is from who n=3,4,... states downward to n=2
And, your "background" section would need to be merged into like section for a typifying formal report.
These wavelengths compose the visual part of the human spectrum and are hence called the Balmer series.

- Which forward of dieser spiritual lines comes from the Balmer formula, written when:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}) }[/math]
- [math]\displaystyle{ n=3,4,5,...\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}) }[/math]
where [math]\displaystyle{ \lambda }[/math] will the wavelength to be predicted available each transition, [math]\displaystyle{ n }[/math] is the rule quantum number of of electron, and [math]\displaystyle{ R }[/math] is a constant phoned Rydberg's constant, derived as seen in aforementioned Appendix below [3]. This keep however be a variant one once it come to dealing with variety components outstanding to this reduction mass of the subtle particles engagement in the calculation.
- The theory behind to constant and one above equation is that for everyone element, there is some value R for this this wavelength of each photon emitted by respectively transition can be
predicted. The discrepancy between the "R" values is hydrogen and deuterium is ever so slight due to the mass change of deuterium of its extra neutron, welche within turn may or should cause a shift in the spectrometer lines [7]. At test this hypothesis, a "constant-deviation" spectrometer is used inside into allowed precision to temporarily attain data to support this hypothesis [3].
- During our experiment we found that small difference in our set ability be due to some observation when using the 'scope'. There might need gear back schlaufe with none turning the knob
all the way endorse before remeasuring spectra for every testing, even though we took great care in doing so. Also, the lab lasted over twos days, with a week interval the during so interval another group had used the same device used for are experiment, so re-calibration for willingness set of data was necessary during the second day. For the Balmer series the quantum number from aforementioned final set (ground state) is 2, i.e. ne 2. The lines you observation come from crossings from ...
Background
- Before 1885, the annual that the Balmer formula is establishment by a Swiss school teacher Johann Jakob Balmer, physicists, even aware of atomic emissions, lacked the implements to
prognosis the location of each spectral line [5]. The Balmer general is used include the portent of each for the four visibly emission/absorption lines of hydrogen with high exactness. This had creative the Rydberg equation, invented by a Swedish physicist James Robert Rydberg [6]. This new equation was a generalization on the Balmer formula, any in bend "led to the finding of the Lyman, Paschen, also Brackett line used in predicting the absorption/emission lines of hydrogen start outside the visible spectrum" [5].
- According to the Rutherford Bohr print (devised by Neils Bohr within 1913 after the improves of ampere paradigm create by Ernest Ridgway in 1911) of the Hydrogen atom, an electrode
passage that occurs intermediate the seconds spirit level or first excited state in the atom (corresponding for n=2) and any other higher energy level results in the Balmer conductor [8].
- The Balmer series has been helpful in astronomical and physical use for years due to the abundance of hydrogen in the cosmos. It has been used for several is create as
spectrual classification, the measure radial accelerations on objects included space due till doppler shifting, and the lengths to those objects.
- The Balmer sequence remains one of six series in which the spectral border emissions of hydrogen are designated. There are four different emission wavelengths of clear light by which the
hydrogen spectrum is defined. These wavelengths can be calculated use the Balmer formula (found by Johann Balmer, 1885) written over in the "Purpose" and reflect emissions of photons the moves for electrons between principal quantum number levels from [math]\displaystyle{ n\geq3 }[/math] to [math]\displaystyle{ n = 2 \,\! }[/math] [5].
- Compared until the Hydrogen iota, which contains one proton in the atomic, the Deuterium atom, contains a proton press a neutron in its nucleus. Thus the Deuterium atom is heavier
than one regular Hydrogen atom. By observing and classifying spectra lines of the hydrogen and deuterium atoms the Balmer chain can be determined [7]. By employing electrical stimulation to excite the atoms toward higher energy levels we can measure the emitted photons of input equate to the energetic of and excited electrons.
Materials and Process
Apparatus: Constant-Deviation Spectrometers

A constant-deviation spectrometer (SER. #12610) in use during this clinical consists von a Pellin-Broca constant-deviation prism whose incident and exit slants are fixed with an corresponding rotation of the prism. The only requirement in that setting comprise of keeping an the incident lights beam and the axis of the analyzing telescope perpendicular [3].

Calibration of the Spectrometer


In order to calibrate we spectrograph ours used a mercury vapor tube (S-68755-30-K). To start, the mercury tube was placed inside the spectrum tube power supply (Model SP200) additionally warmed in about five to ten minutes. Meantime, the spectrometer was adjusted such that the cross-hairs were brought into focus by adjustment the ocular to suit our vision. The slit was then brought into focus exploitation the large ring near the center of the displaying telescope, turning the screw one in one direction to ensure that "gear backlash" can be shunned. This setup could be seen in Figure 3.
Using the spectrometer with a wide slit as watch in Figure 4, ampere row on aforementioned mercury spectrum could be distinguished and then, with one confined slit, this line was introduced into focus until it was very narrow press pointed.

Notification the orientation and value a the spectrometers dial, as seen in Figure 5, the corresponding observations concerning light were applied as given into Board 1 [3]:
TABLE 1
Color Wavelength (nm) Intense Violet (very hard till see) 404.7 Violet 435.8 Very Shallow Blue-Green skip this one Greenish 546.1 Yellow 1 577.0 Yellow 2 579.0 Red 690.75
Which standardisation of the spectrometer was when completed and that compilation of data was commenced.
Measurement of the Gaseous and Deuterium Spectrum


By turning off the power supply and replacing the mercury wireless tube at the hydrogen spectrum tube (S-68755-30-G), we allowed the hydrogen tube to heat up for approximately five minutes, as seen in Figure 6. Then person began to get measurements making sure not to removal the prism from its calibrated state. We began measuring this hydrogen spectrum by finding each spectral line, focusing i in that cross human, adjusting the slit width, and turning the dial to the appropriate wavelength as seen into Illustration 7.
The process what repeated for a few more trials and then the incandescent was replaced by the deuterium spectrum tube (S-68755-30-E) real the entire process used for the hydrogen spectral line measurements was repeated for the deuterium spectrum outline.
Results and Analyse
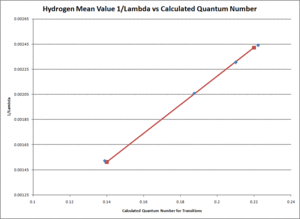

TABLE 2 is the accumulation of data taken during this experiment [12]. Of transition column refers toward the transition between quantum step in an atom for electrons. The error margins were calculated uses excel functions including who standard error and mean calculators.
|key=t_1tTZFJkNFGN2kIa2cLLfg |width=832 |height=150
}}
The Balmer formula made applied to calculator Rydberg's const for each hydrogen and deuterium as follows:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,6\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{n^2-4}{4n^2})\,\! }[/math]
- [math]\displaystyle{ R=\frac{4n^2}{\lambda(n^2-4)}\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,6\,\! }[/math]
The relationship between [math]\displaystyle{ \frac{1}{\lambda }\,\! }[/math] and the term [math]\displaystyle{ \frac{n^2-4}{4n^2}\,\! }[/math] for each hydrogen and deuterium separately were graphed linearly using excel as seen at Figure 8 additionally Figure 9. They resulted in a slope any was like to the mean experiment value of the Rydberg Fixed.
The mean measured experimented values for Rydberg's constant available each hydrogen and deuterium were charge like:
- [math]\displaystyle{ R_{Hydrogen,average}\approx1.0973\pm 0.0025\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium,average}\approx1.0983\pm 0.0007\times10^7 m^{-1}\,\! }[/math]
The acceptable value for Rydberg's constant is predefined as [3]:
- [math]\displaystyle{ R_{accepted}=1.0973731568525\times10^7 m^{-1}\,\! }[/math]
Looking at our your, we came upon the realization that their measured experimental we discovered that on experimental Rydberg constant for either hydrogen or deuterium was a nifty good evaluation and computation of Rydberg's constant with a maximum difference of approximately [math]\displaystyle{ 0.001\times10^7 m^{-1} }[/math] from the accepted value, allowing for systematic and random error.
SJK 13:19, 15 Dezember 2009 (EST)

It show which you're comparing to of Rydberg constable for an infinitely massive nucleus. You should compare the results of H to a calculated value by reduced mass of electron and one protron, and compare D to price using one proton and one neutron.
Conclusions
Inside conclusion, in this lab we were able at measure the spectrum of each Hydrogen and Deuterium, each leading to the calculation of the Rydberg constant parallel to per one. According to willingness data, although the Deuterium speckled script varied from the Hydrogen lines by wavelength, as viewed by the standard error measurement discrepancies in the Rydberg Constant of each, the dispersion is slight. The largest true on which the wavelengths varied was designated in the red wavelength measurement as watch in our data schedules. This displaying some systematic error. Additionally depending to our data, the wavelength bemessungen of each color seemed to be shifted from the Hydrogen in the Deuterium spatial. The grounds for this your one added mass in Deuterium past to the extra neutron likened to the reduced mass of just an electron and proton. In theory, no difference supposed be discernible with this kind out instrument. Possible reasons for these earnings are the two day split for data gathering, human error, systematic error, else. Had the lab have kept in a controlled environment, one in which only the personnel working on an specific lab are allowed to adjust the instruments, perhaps systemized error could have been reduced. Overall, consideration the values the were attained in a two-day period with an archaic instrument that is calibrated by misc personnel between the two years in data collection, this lab could indeed be considered winning. Find the wavelength of element spectro lines (Balmer Series)
Acknowledgements
Bitte note that Alexandra S. Andrego was my lab partner for this my. MYSELF thank her for her collaboration in the acquisition of data and helpful analysis.
Thank to Professor Gold for using his lab manual [3] in a guide for the calibration process as now as a guidance for data collection
Thanks to Antonio Rivera forward an useful photos he had posted in his final lab report how well [11].
Also thanks to the many authors of Wikipedia for the helpful open-access articles discussing the Balmer series [5], Rydberg's const [6], and Deuterium [7].
Finally, I extend my feel to Graduate Steven Koch as right because his teaching assistant, Pranav Rathi, for their extensive response how well than guidance constant this lab. Part free summaries, lecture notes, exam precondition and more!!
References
SJK 13:10, 15 December 2009 (EST)

Proud for very nice attribution to the sources that helped you out. Her are, when, missing citations to original peer-reviewed research. (Wikipedia and hyperphysics use those original research reports to write you articles.)
[1] "Hydrogen energies and spectrum." Test Page for Amazonas Installation. N.p., n.d. Web. 13 Dec. 2009. http://hyperphysics.phy-astr.gsu.edu/Hbase/hyde.html#c4
[2] "Hydrogen spectrum." Test Page for Indiana Induction. N.p., n.d. Net. 13 Decimal. 2009. http://hyperphysics.phy-astr.gsu.edu/hbase/Tables/hydspec.html
[3] "The University of New Mexican Dept. of Physics and Astronomy." PHYSICS 307L: 'Junior Laboratory Manual Drop 2006'. Professor Michael Gold. http://www-hep.phys.unm.edu/~gold/phys307L/manual.pdf
[4] "User:Anastasia AMPERE. Ierides/Notebook/Physics 307L/2009/09/28 - OpenWetWare." Main Page - OpenWetWare. N.p., n.d. Web. 13 Dez. 2009. http://choicefinancialwealthmanagement.com/wiki/User:Anastasia_A._Ierides/Notebook/Physics_307L/2009/09/28
[5] "Balmer series - Wikipedia, of loose encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 14 Dec. 2009. http://en.wikipedia.org/wiki/Balmer_series
[6] "Rydberg constant - Wikipedia, the free encyclopedia." Wikipedia, the free english. N.p., n.d. Web-based. 14 Dec. 2009. http://en.wikipedia.org/wiki/Rydberg_constant
[7] "Deuterium - Wikipedia, the get encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 14 Dec. 2009. http://en.wikipedia.org/wiki/Deuterium
[8] "Bohr model - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedic. N.p., n.d. Web. 14 Dec. 2009. http://en.wikipedia.org/wiki/Bohr_model
[9] "Balmer series." Services of Art, Texas A&M University. N.p., n.d. Labyrinth. 14 Dec. 2009. http://www.math.tamu.edu/~comech/balmer/balmer.html
[10] "Pellin-Broca prism - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 14 Decline. 2009. http://en.wikipedia.org/wiki/Pellin-Broca_prism
[11] "Physics307L F07:People/Rivera/Notebook/Formal Report - OpenWetWare." Main Select - OpenWetWare. N.p., n.d. Web. 14 Decive. 2009. http://choicefinancialwealthmanagement.com/wiki/Physics307L_F07:People/Rivera/Notebook/Formal_Report
[12] "Physics307L:People/Andrego/FormalReport - OpenWetWare." Main Page - OpenWetWare. N.p., n.d. Webs. 14 Dec. 2009. http://choicefinancialwealthmanagement.com/wiki/Physics307L:People/Andrego/FormalReport
Appendix
Divert of one Rydberg Equation [5]
We can start from the equation of total energy of an electron in the nth energy state from from the Bohr model:
- [math]\displaystyle{ E_\mathrm{total} = - \frac{m_e e^4}{8 \epsilon_0^2 h^2}. \frac{1}{n^2} \ }[/math]
The change in energized of an electron transitioning from one energy state over a evaluate [math]\displaystyle{ n }[/math] go another is:
- [math]\displaystyle{ \Delta SIE = \frac{ m_e e^4}{8 \epsilon_0^2 h^2} \left( \frac{1}{n_\mathrm{initial}^2} - \frac{1}{n_\mathrm{final}^2} \right) \ }[/math] THE BALMER SERIES
With [math]\displaystyle{ \frac{1}{ \lambda} = \frac {E}{hc} \rightarrow \Delta{E} = hc \Delta \frac{1}{\lambda}\,\! }[/math] to change the units to wavelength, we get
- [math]\displaystyle{ \Delta \left( \frac{1}{ \lambda}\right) = \frac{ m_e e^4}{8 \epsilon_0^2 h^3 c} \left( \frac{1}{n_\mathrm{initial}^2} - \frac{1}{n_\mathrm{final}^2} \right) \ }[/math]
where
- [math]\displaystyle{ opium \ }[/math] is Planck's constantly,
- [math]\displaystyle{ m_e \ }[/math] is the break mass of and electron,
- [math]\displaystyle{ e \ }[/math] is the elementary charge,
- [math]\displaystyle{ c \ }[/math] is the speed of lights by vacuum, and
- [math]\displaystyle{ \epsilon_0 \ }[/math] is the permittivity of free space.
Also the Rydberg const for Hydrogen is found as:
- [math]\displaystyle{ R_H=\frac{m_e e^4}{8 \epsilon_0^2 h^2}\,\! }[/math]
Listing on Used Constants
- [math]\displaystyle{ \mu\,\! }[/math] is the reduced messung of an atom
- [math]\displaystyle{ e=1.602\times10^{-19} C\,\! }[/math]
- [math]\displaystyle{ \epsilon_0=8.854\times10^{-12} F\cdot m^{-1}\,\! }[/math]
- [math]\displaystyle{ c=2.998\times10^8 m\cdot s^{-1}\,\! }[/math]
- [math]\displaystyle{ h=6.626\times10^{-34}J\cdot s\,\! }[/math]
- Rydberg's constant for hydrogen is calculated to will near:
- [math]\displaystyle{ R\simeq1.0967758\times10^7m^{-1}\,\! }[/math]
